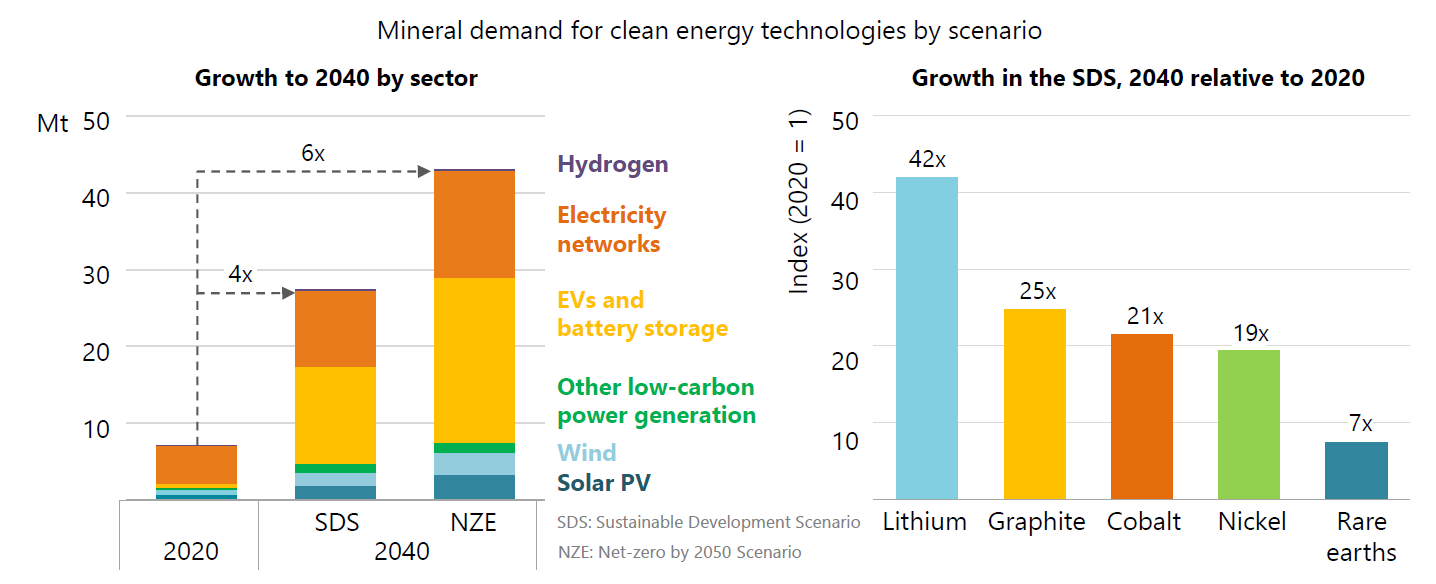
As countries strive to meet the Paris climate agreement goals, the demand for energy transition metals (incl. cobalt) is going to be turbo-charged the coming decades. As pointed out recently in the International Energy Agency’s iconic report “The Role of Critical Minerals in Clean Energy Transitions” the demand for cobalt will grow 21-fold by 2040. Indeed, high-purity cobalt is of vital importance for most types of Li-ion battery applications. However, it is challenging to produce such “battery-grade” cobalt. Elaborate solvent extraction flowsheets are often used to separate cobalt from its impurities. Nevertheless, these flowsheets require extensive optimisation before high-purity cobalt is finally obtained. SOLVOMET/SIM² KU Leuven researchers Rayco Lommelen and Koen Binnemans combined the chemistry of cobalt solvent extractions by a basic extractant in a thermodynamic model. Their paper shows the first steps towards a more predictive approach to calculating solvent extraction outcomes, which will accelerate the design of processes to produce high-purity cobalt. The work was published in ACS Omega.
Figure 1: Demand for critical minerals is set to soar over the next two decades as the world pursues net zero goals; overall requirements rise by as much as 6 times, but individual minerals, led by lithium, rise even faster (image courtesy of IEA "The Role of Critical Minerals in Clean Energy Transitions – Launch presentation, Paris, 5 May 2021)
Investigated SX system
In the present work the SOLVOMET/SIM² KU Leuven researchers investigated a solvent extraction process where cobalt is extracted with a basic extractant similar to Aliquat 336 in chloride media. The addition of salts or acids is necessary to transfer the desired metals from the feed solutions to the organic phase that contains the basic extractants. The anions of the salt or acid combine with the metal cations to form extractable metal complexes.
However, adding a lot of salt or acid to a feed solution changes its chemistry significantly. That makes it difficult to calculate the extraction chemistry with simple chemical reactions. The only solution seems to incorporate all the effects of the added salts or acids in a computational model that can also describe the rest of the solvent extraction chemistry.
Thermodynamic framework
A suitable thermodynamic framework should be selected for the construction of the cobalt solvent extraction model. It should have a sound chemical basis to make a predictive model that can calculate aspects of a solvent extraction that deviate (slightly) from the input data used to construct the model.
Such chemically-based models can also give the user more insights into the chemistry behind the solvent extraction process. However, purely theoretical frameworks are difficult to use for the calculation of real-world solvent extraction processes. They require data that is often not readily available and have difficulties describing complex solutions with high concentrations of salts and acids.
Therefore, the authors chose the MSE-OLI framework of OLI Systems. It can be positioned somewhere in the middle between empirical and theoretical frameworks. The major drawback of OLI-MSE is the absence of a description for the formation of micro-emulsions or reversed micelles in the organic phase. This limits the applicability of the current solvent extraction model to a fixed extractant concentration.
Cobalt solvent extractions
The model for the extraction of cobalt by a basic extractant is obtained by combining speciation data of the metal complex gathered by UV-VIS absorption spectroscopy, composition data of both phases, and the water activity of the system. The effects of different kinds and amounts of salts on the extraction behaviour of cobalt arise automatically in the model. This results in an accurate calculation of the extraction efficiency of cobalt from different salts.
This work comprises the first step to really calculate the complex chemistry of solvent extractions by basic extractants, although further optimisation is still necessary.
Full reference paper
Lommelen, R.; Binnemans, K. Thermodynamic Modeling of Salting Effects in Solvent Extraction of Cobalt(II) from Chloride Media by the Basic Extractant Methyltrioctylammonium Chloride. ACS Omega 2021, 6 (17), 11355–11366. https://doi.org/10.1021/acsomega.1c00340.
Acknowledgements
The authors thank the FWO Flanders (project G0B6918N) for financial support. The research was supported by the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Programme: Grant Agreement 694078— Solvometallurgy for Critical Metals (SOLCRIMET). The contents of this publication are the sole responsibility of the authors and do not necessarily reflect the opinion of the European Union.
Want to know more about the SX work performed in the SOLVOMET (SIM² KU Leuven) Research Group? Check out the SOLVOMET corporate presentation…
 SOLCRIMET Advanced ERC Grant
SOLCRIMET Advanced ERC Grant